Janssen’s Carvykti (cilta-cel) FDA approval sets the pace for a cell therapy race in multiple myeloma
Second-to-market Carvykti is expected to overtake BMS’ Abecma by 2023 due to a stronger clinical profile.
The FDA has approved Janssen’s and Legend Biotech’s BCMA-targeting CAR T-cell therapy Carvykti (ciltacabtagene autoleucel; cilta-cel) for the treatment of adults with RRMM after four or more prior lines of therapy, on its target date of February 28, 2022, after a three-month PDUFA resolution.
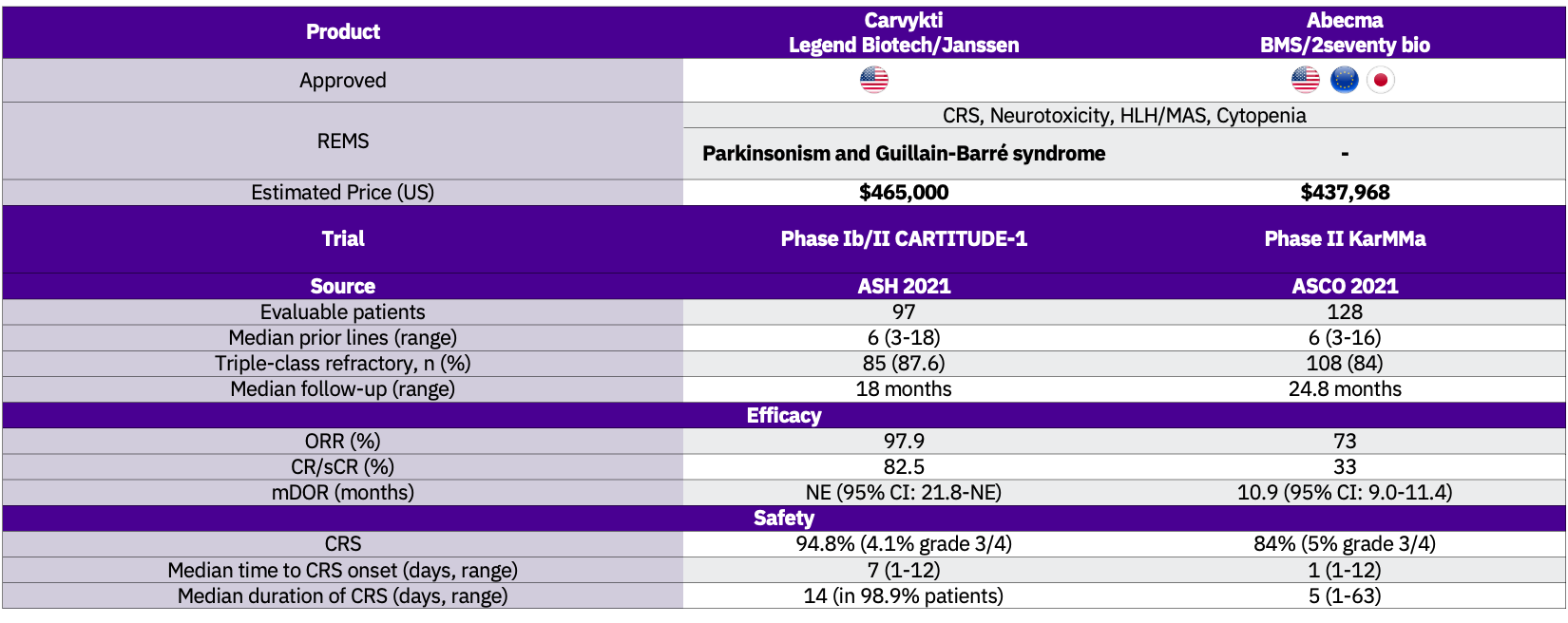
Carvykti is the second CAR T approved for the treatment of multiple myeloma, following BMS’ and 2seventy bio’s Abecma (idecabtagene vicleucel; ide-cel) FDA approval in March 2021. Both assets target BCMA, share the same indication and are available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS).
Carvykti’s approval is based on data from the pivotal Phase I/II CARTITUDE-1 study (NCT03548207) with a 97.9% ORR, at a median of 18 months follow-up. Carvykti is superior to Abecma in terms of efficacy but exhibits a higher incidence of CRS, grade 3/4 neutropenia, and CAR-T cell-related neurotoxicity compared to Abecma.
Manufacturing challenges have been severely affecting Abecma’s supply. Janssen and Legend Biotech are addressing that by activating a limited network of certified treatment centres to scale its production capacity throughout the US in 2022.
Our market share uptake simulation places Carvykti in a strong position upon entry compared to Abecma, based on Carvykti’s greater clinical responses and reflected in positive KOL sentiment. In the launch year we expect Carvykti to hold 7.1% market share in 4L+ increasing to 12.9% at peak share in 2023 (annual revenue estimated at $365 million). From 2023 we expect Carvykti market share to drop due to the entry of “off-the-shelf” BCMAxCD3 bispecific antibodies, which boast impressive efficacy and lower incidence of neurotoxicities.
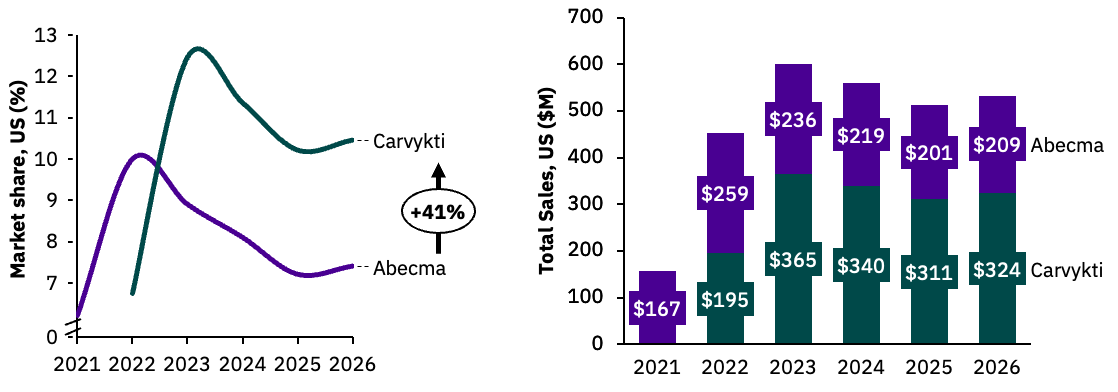
Source(s): Janssen Carvykti Approval Press Release; Abecma Cost; Abecma FDA label; Abecma REMS; EndpointsNews; Airfinity
For more information about Airfinity's analytics and predictive capabilities in Multiple Myeloma and beyond, contact the team here.