Pandemic focuses the need for a data-driven antiviral strategy
BY ARSALAN AZAD
The SARS-CoV-2 pandemic has uniquely highlighted the dearth of antiviral compounds that can be readily mobilised and deployed for the treatment of viral diseases. There are currently only 10 clinically approved candidates available for the 220 viruses known to infect humans. Antiviral drug discovery has thus far been slow and reactive.
We are currently tracking over 245 antiviral candidates under investigation for COVID-19 treatment, the majority of which remain in preclinical studies (150) (Fig. 1).

Fig. 1. A summary of the stage of COVID-19 antiviral development and the Airfinity priority classification
Five of these antivirals are currently approved for COVID-19 treatment globally (Fig. 2).
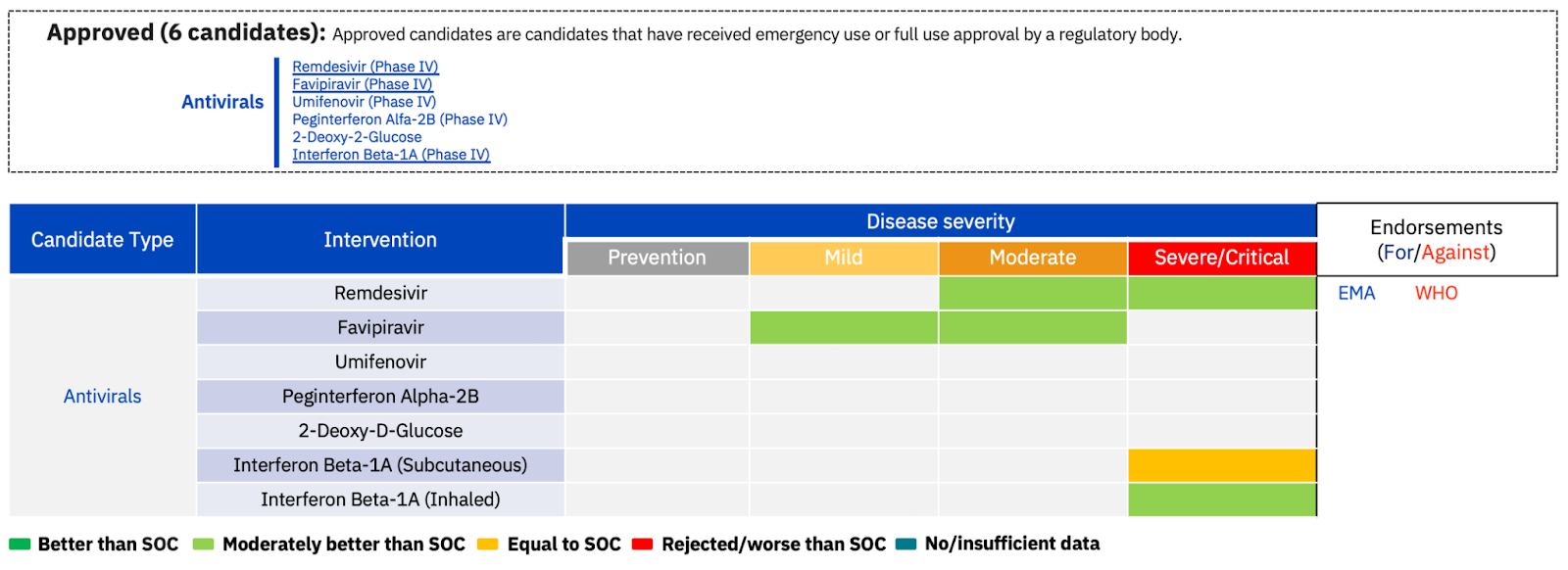
Fig. 2. An overview of the 6 currently approved antiviral COVID-19 treatments
It is thought that combination antiviral therapy, comprising different mechanisms of action to target multiple stages of a virus life cycle, will be the most effective; with direct-acting antivirals offering the best protection against specific viruses. Nevertheless, the best long-term strategy for protection against future outbreaks resulting from unknown viral families is likely the development of host-targeted antivirals, as many manipulate common host cell processes in response to multiple viruses. In the case of COVID-19, most antivirals (48%) are direct-acting, with remaining either being host-targeting (21.5%) or unconfirmed as of yet.
The most common mechanism of action being investigated for both direct-acting and host-targeted antivirals are the protease inhibitors, which are essential for viral entry (via cleavage of the SARS-CoV-2 spike protein) and maturation of infectious viruses that can infect other cells or people (Fig. 3).

Fig. 3. The most common mechanisms of action being investigated for direct-acting and host-targeted antivirals for COVID-19 treatment
In either case, antivirals can not be developed against all potential viral threats and thus a concerted effort to understand which viruses have the greatest epidemic potential is needed. COVID-19 has identified a way in which advanced structural biology, viral phenotyping, enrollment onto multi-arm platform trials can expedite the development of such therapeutics. The time to approval for antiviral drugs has now been expedited from an average of 82 months (for non-COVID-19 indications) to 16 months (for COVID-19) (Fig. 4).

Fig. 4 Comparison of clinical development timelines for Remdesivir, the first and most widely approved antiviral therapy for COVID-19.