Airfinity Launches an RNA Therapeutics and Vaccine Intelligence Tool
Airifinity has launched an RNA Treatments and Vaccines Platform providing insights and a holistic overview of the whole RNA landscape monitoring candidates and delivery system technology from preclinical to clinical and through to commercialisation. The platform tracks candidates, clinical trials, delivery systems, RNA manufacturing sites and capacities, a delivery system demand model, new approvals, new deals, media and press releases, as well as company interactions such as licensing agreements, partnerships, and M&A.
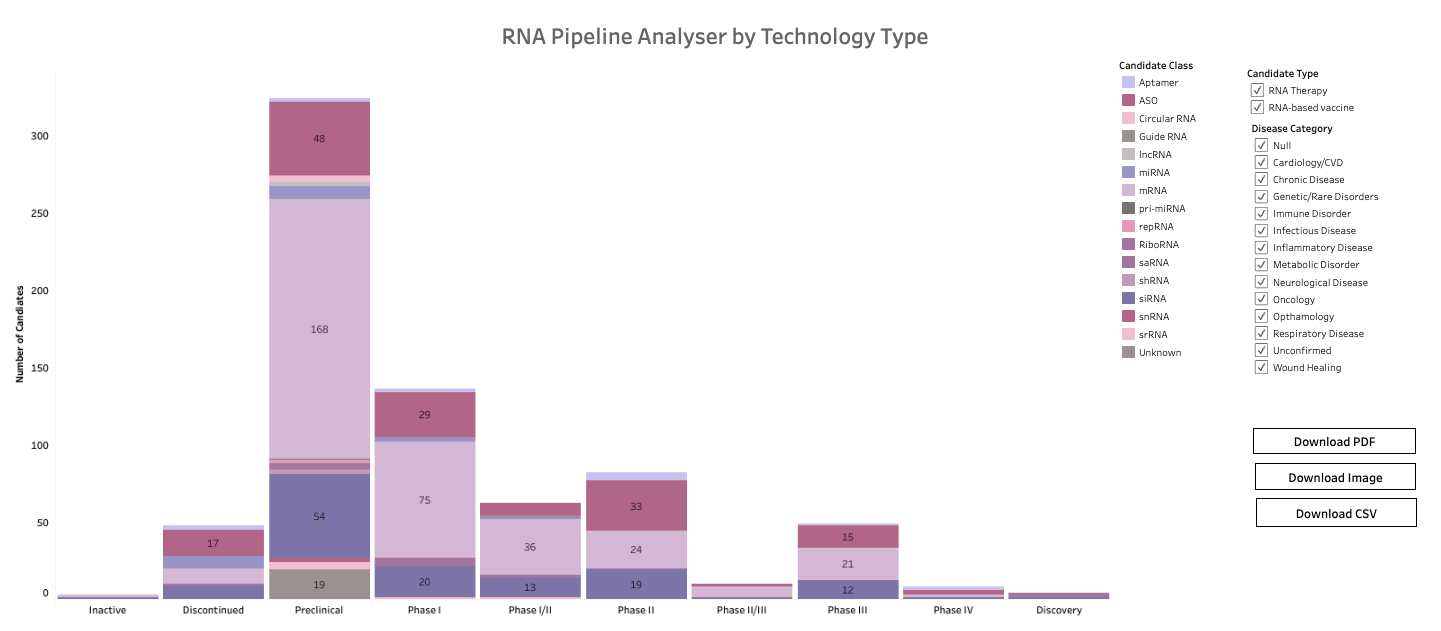
What is Airfinity’s RNA intelligence platform?
→ An overview of 700+ candidates in development and changes to the candidate clinical and preclinical landscape.
→ RNA Manufacturing Map including HQ, R&D, CDMO, CRO, and CMO sites
→ Material type over time for GMP grade RNA candidates to predict future demand.
→ Clinical trial overviews including new locations and announced timelines of completion.
→ New approvals and expedited status designations.
→ New funding deals.
→ A network interaction map of how companies within the RNA space interact (licensing agreements, partnerships, collaborations, mergers and acquisitions).
→ Media and press releases.
→ RNA facilities map including HQ, R&D, CDMO, CRO, and CMO sites.
→ An overview of generic and proprietary delivery system technologies in development.
To request a free demo of the platform and speak with our specialist team, get in touch here.